
On October 26, 2023,

During the testing procedure, we selected suitable alternative contaminants in agreement with the EFSA guidelines (EFSA CEF Panel, 2011) and in accordance with the recommendations of the US Food and Drug Administration (FDA, 2006). These alternative contaminants included different molecular masses and polarities to comprehensively cover the relevant contaminant categories while proving to be suitable for monitoring the behavior of PET in the recycling process.
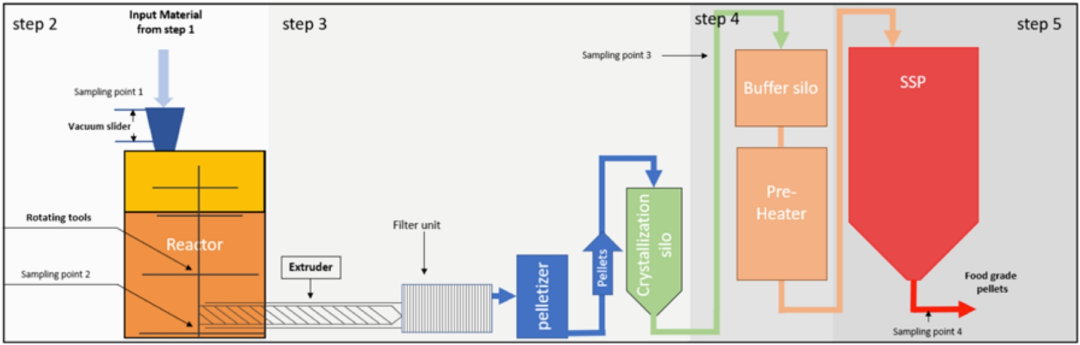
Multiple batches of contaminated PET bottle flakes were used for storage and agitation under specific conditions, and subsequently, the contaminated flakes were rinsed with specific concentrations of ethanol and tested for decontamination efficiency, while for Vacunite (

After completing multiple challenge procedures, the data provides intuitive evidence that



Therefore, the recycled PET obtained from this process is not of safety concern, when used at up to 100% for the manufacture of materials and articles for contact with all types of foodstuffs, including drinking water, for long-term storage at room temperature or below, with or without hotfill.




01 R-PET sheet FDA (NOL)

PCR-PET Sheet Obtains the FDA No Objection Letter
FDA Official No. PNC2628
Range of use: E-G
02 R-PET pellet FDA (NOL)
In August 2022, Guolong's R-PET flakes (pellets) received A-H Whole Factory Approval from the U.S. FDA; it was the pioneer to pass the U.S. FDA's official laboratory challenge test in the Asia-Pacific region.
Since September 2020, Guolong PET flakes have been undergoing more than one year of challenge testing in the official laboratory of the FDA in the U.S., from recycling bottles to cleaning bottles and flakes, extrusion pelletizing, SSP, hazardous substance addition and subsequent migration tests and challenge tests. The FDA audited Guolong's process and its ability to remove contaminants in the cleaning and recycling processes, as well as its operational plans for challenge testing and migration tests.
The FDA has certified that Guolong Recycling's post-consumer PET material can be used in the production of PET containers with up to 100% recycled content under A-H conditions. Guangxi Guolong Recycling is the pioneer food-grade R-PET producer in China to get the certification of the whole plant under A-H applicable conditions.

PCR-PET Pellets Pass FDA Challenge Test, Obtain FDA No Objection Letter
FDA Official No. PNC2783
Range of use: A-H
03 R-HDPE Pellet FDA (NOL)
In March 2022, Guolong's R-HDPE pellets received an official No Objection Letter (NOL) from the U.S. FDA; these post-consumer recycled and re-produced R-HDPE pellets can be used for food packaging, cosmetic packaging, and other applications.

PCR-HDPE Pellet Obtains the FDA No Objection Letter
FDA Official No. PNC2752
Range of use: E-G
04 R-PP Pellet FDA (NOL)
On June 6, 2023, Guolong Recycling received a No Objection Letter (NOL) from the U.S. FDA for food contact materials, confirming that its secondary recycling process to produce post-consumer recycled polypropylene (PCR-PP) is suitable for food contact applications.

PCR-PP Pellets Pass FDA Challenge Test, Obtain FDA No Objection
FDA Official No. PNC2752
Range of use: E-G